Video
1 / 6
cTnI Troponin I Test Rapid Test Card
$0.50
~ $0.74
/ Piece/Pieces
Options:
- cTnI Troponin I Test Rapid Test
- cTnI Troponin I Test Rapid Test
- cTnI Troponin I Test Rapid Test
- cTnI Troponin I Test Rapid Test
- cTnI Troponin I Test Rapid Test
- cTnI Troponin I Test Rapid Test
Send Inquiry
| Model No. : | cTnI Troponin I Test Rapid Test |
|---|---|
| Brand Name : | MR |
| Brand Name : | MR |
More
2yrs
Changchun, Jilin, China
- Manufacturer
- Trade Company
- OEM service
- Gold Supplier

- Platform Certification


Changchun ZYF science and technology CO.,LTD
You might also like
Product description
Product Description
Specification
|
item
|
Troponin I Test
|
|
Place of Origin
|
China
|
|
|
Shandong
|
|
Brand Name
|
MR
|
|
Model Number
|
cTnI
|
|
Power Source
|
Manual
|
|
Warranty
|
2 years
|
|
After-sale Service
|
Online technical support
|
|
Material
|
plastic, paper
|
|
Shelf Life
|
2 years
|
|
Quality Certification
|
ISO13485
|
|
Instrument classification
|
Class II
|
|
Safety standard
|
MSDS
|
|
Product name
|
cTnI Test Cassette
|
|
Format
|
Cassette
|
|
Dimension
|
4.0mm
|
|
Specimen
|
Whole blood/serum/plasma
|
|
Package
|
20 pcs/box
|
|
Storage
|
4-30°C
|
|
Shelf Life
|
24months
|
|
Method
|
Immunochromatography
|
|
MOQ
|
1000pcs
|
|
Brand
|
MR
|
Intended Use
cTnI is released rapidly into blood stream soon after the onset of acute myocardial infarction (AMI). Levels of cTnI remains elevated for up to 6-10 days. The level of cTnI is below 0.06ng/ml in average in health people, and also not detected in the patients with skeletal muscle injury. Therefore, cTnI is a specific marker for diagnosis of AMI patients. The Rapid Troponin I Test is Gold Immunochromatography Assay for the qualitative determination of cTnI in human whole blood,serum or plasma.
Test procedure
Instructions must be read entirely before taking the test. Allow the test device controls to equilibrate to room temperature for 30 minutes (20℃-30℃) prior to testing. Do not open the inner packaging until ready, it must be used in one hour if opened (Humidity: 20%~90%, Temp: 10℃-50℃) Strip: 1. Remove the test device from the sealed pouch, put the end of the test strip print with arrow into the sample, the interface of serum/plasma should not exceed the max line, take it out and place the test device on a clean and level surface after 15 seconds.
2. Observe the test results immediately within 15minutes, the result is invalid over 15 minutes. Cassette: 1. Take off the outer packing, put the cassette onto the desk with the sample window up.
2. Drop 3 drops (100ul) of serum or plasma vertically into the sample hole of cassette.
3. Observe the test results immediately within 15 minutes, the result is invalid over 15 minutes.
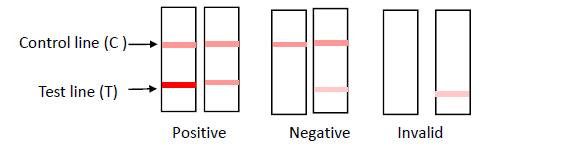
INTERPRETATION OF RESULTS
POSITIVE: Two distinct red lines appear. One line should be in the control region (C) and the other line should be in the test region (T). NEGATIVE: One red line appears in the control region(C). No apparent red or pink line appears in the test region (T). INVALID: No red lines appear or control line fails to appear, indicating that the operator error or reagent failure. Verify the test procedure and repeat the test with a new testing device.
Manufacturing procedure


Company Profile
Established in Year 2014,CHANGCHUN ZYF SCIENCE AND TECHNOLOGY Co., Ltd. is a high-tech enterprise devoted to the R&D, manufacturing, and sales of high-quality diagnostic products including laboratory equipment and clinical diagnostic reagents.
We can offer more than 30 kinds of urine analysis strips sold well and enjoyed high reputation from both domestic and overseas markets. Based on the principle of "customer first, quality first", our products continue to cover world-wide markets with the advantages of stable performance and high accuracy.
We registered MR International Healthcare Technology Co., Ltd. in Hong Kong for overseas sales. Through MR, we also possess some manufacture facilities in Zhejiang, Guangdong and Jiangsu provinces of China in order to help reduce the cost while caring about the quality.
We are committed to provide most reliable medical devices and solutions to people in every corner of the earth. Accomplishing the mission of "maximizing the reliability of medical products" is our goal. We do care about obtaining the trust from our partners and patients. We are dedicated to innovation in the fields of Rapid Test, POCT, In-Vitro Diagnostics, and Medical Consumables, such as vacuum tubes, tube holders, etc.
Customer Photos


Certifications


Video
2yrs
Changchun, Jilin, China
- Manufacturer
- Trade Company
- OEM service
- Gold Supplier

- Platform Certification

Send your inquiry to this supplier
Send Inquiry

























