Video
1 / 4
drug (BAR) test kits-Cassette
Options:
- 2.5mm strip
- 3mm strip
- 3.5mm strip
- 4mm cassette
- 3mm cassette
- 4mm cassette
| Model No. : | BAR-S |
|---|---|
| Brand Name : | Contory |
| place of origin : | China |
Changchun, Jilin, China
- Manufacturer
- Trade Company
- OEM service
- Gold Supplier

- Platform Certification


Product description
Product description
Product details

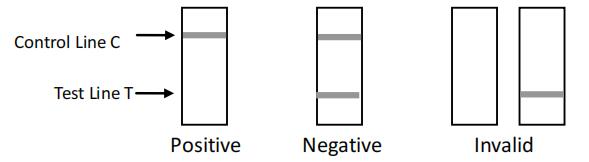
INTERPRETATION OF RESULTS
Production procedure

Application

Company Overview
Jilin Sinoscience Technology Co., Ltd. is a high-tech enterprise specializing in the research, production, sales and service of Laboratory Diagnostic products. We were established in Changchun in 2021. Our main products are in vitro diagnostic reagents and instruments,including COVID-19 Medical Products,Medical Instruments And Equipment, Laboratory Diagnostic,Medical Consumables. We have more than 70 reagents in the field of IVD and continue to develop automatic chemical analyzers, urine analysis products, etc. We are committed to becoming one of the best suppliers in the IVD industry, providing various series of products with advanced technology and first-class quality. We have more than 6 production lines which are urine analyzer series,chemistry analyzer series,hematology analyzer series,Reagent Strips for Urinalysis series,rapid tests series,COVID-19 Medical Products series.At the same time, we also hope to become a third-party medical testing center that provides regional medical testing services, equipped with self-developed regional LIS system, modern disinfection center, cold chain logistics system, cloud platform and other supporting systems.


Exhibition

Workshop

Certificate

Transportation

Welcome to inquire

Video
Changchun, Jilin, China
- Manufacturer
- Trade Company
- OEM service
- Gold Supplier

- Platform Certification

Send your inquiry to this supplier