Video
1 / 7
Hydrochloric Acid 31% 32% For Water Treatment
Options:
- hydrochloric acid 33%
- hydrochloric acid 32%
- hydrochloric acid 31%
- hydrochloric acid 35%
- hydrochloric acid 37%
| classification : | Hydrochloric Acid |
|---|
Wuhan, Hubei, China
- Agent
- Distributor/Wholesaler
- Manufacturer
- Service
- Trade Company
- Platform Certification

- SGS Certification

Product description
Hydrochloric acid can be produced by several methods. It is obtained from the reaction of sodium chloride and sulfuric acid in a cast iron retort at elevated temperature. Although reaction starts at 150°C, the complete reaction occurs at about 600°C:
2NaCl + H2SO4→ Na2SO4 + 2HClHydrochloric acid also is made by the Hargreaves process in which a mixture of salt, sulfur dioxide, oxygen, and water are heated at elevated temperatures, between 430 to 540°C.
Hydrochloric acid is classified as inorgenic chemicals which is kind of inorgenic acid, its solution is a colorless watery liquid with a sharp, irritating odor. Consists of hydrogen chloride, a gas, dissolved in water. Sinks and mixes with water. Produces irritating vapor. Hydrochloric acid used in the production of chlorides, fertilizers, and dyes, in electroplating, and in the photographic, textile, and rubber industries. Hydrochloric acid is corrosive to the eyes, skin, and mucous membranes.
Basic Information:
CAS: 7647-01-0
Molecular Formula: HCl
Molecular Weight: 36.4609
Specific Gravity 1.19
Melting point -35 °C
Boiling point >100 °C (lit.)
Density 1.2 g/mL at 25 °C (lit.)
vapor density 1.3 (vs air)
vapor pressure 613 psi ( 21.1 °C)
refractive index 1.3535
Flash point 10℃ (tag closed test)
storage temp. Store at +2°C to +25°C.
solubility H2O: soluble
form: Light Yellow liquid
History
Basilus Valentinus of Italy was first to isolate the acid and reported it under the name spiritus salis in the fifteenth century. Glauber prepared this acid by the reaction of sulfuric acid with common salt in 1648. Lavoisier proposed the name muriatic acid in 1789 after muriate, the term referring to a chlorine-containing inorganic substance. Sir Humphrey Davy proved the gas was composed of only hydrogen and chlorine in 1810. Subsequently, the gas was named hydrogen chloride.
Dilute hydrochloric acid occurs in the stomachs of mammals. Gaseous hydrogen chloride occurs in trace concentrations in the atmosphere.
Specifications:
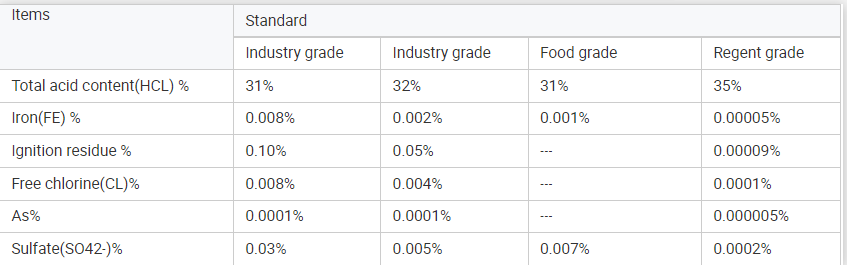
Products Packing:

Uses

Hydrochloric acid is one of the most important industrial chemicals and has numerous applications. Both anhydrous hydrogen chloride and aqueous acid are used to produce a large number of chloride salts. The acid also is a common laboratory reagent. Some major applications of hydrochloric acid include processing of ores and extraction of metals from their minerals; in metal cleaning, particularly in steel pickling to dissolve oxide impurities; production of alumina, titanium dioxide, and other metal oxides by various hydrometallurgical processes; production of hydrogen; synthesis of chlorine dioxide; removal of heavy metal impurities from carbon black; activation of bentonite clays; etching of concrete surfaces for finishing operations; and as a catalyst in several organic reactions such as inversion of sugar, hydrolysis of starch to obtain sugar syrup, and esterification of aromatic acids.
Anhydrous hydrogen chloride gas is used to produce phosphonium chloride, PH4Cl, which is a flame retardant for cotton textiles. Other major applications include manufacture of a number of high purity metal chlorides, ammonium chloride, chlorosulfuric acid; recovery of waste metals; preparation of alkyl chlorides and chloroacetic acids; and as a chlorinating agent in organic syntheses.
Rubber hydrochloride, which results from the treatment of natural rubber with hydrogen chloride, can be cast in film from solutions. Such rubber hydrochloride films provide a strong, water resistant packaging material for meats and other foods, paper products, and textiles.
Company Profile:
Wuhan Ruisunny Chemical Co.,Ltd was established in 2010 with registered capital of CNY 20 million, the company focuses on trading of such chemicals as WATER TREATMENT CHEMICALS, FOOD ADDITIVES and general INORGANICS/ORGANICS. Among our main products are: Calcium Hypochlorite, Trichloroisocyanuric Acid,Hydrogen Peroxide
,Ammonium Bicarbonate,Sodium Hydroxide,Organic Silane. Over the years of development,the sales of the company has extended to more than 50 countries or regions. Based on principle of Co-win, we are looking forward to cooperating with you!
Wuhan Ruisunny Chemical Co.,Ltd was established in 2010 with registered capital of CNY 20 million, the company focuses on trading of such chemicals as WATER TREATMENT CHEMICALS, FOOD ADDITIVES and general INORGANICS/ORGANICS.
Video
Wuhan, Hubei, China
- Agent
- Distributor/Wholesaler
- Manufacturer
- Service
- Trade Company
- Platform Certification

- SGS Certification
Send your inquiry to this supplier

















