Video
1 / 4
Nasopharyngeal DNA cell collection flocked swab
| Brand Name : | JSHXRT |
|---|---|
| place of origin : | China |

Product description
This product is produced by Japan's new nylon flocking technology, which is an international new patented production technology. A large number of clinical trials abroad have shown that compared with ordinary sterile swabs, nylon flocking swabs have better effects on the collection and transportation of clinical biological samples, especially for those samples that cannot be sent for inspection in time and have been placed for too long. . We sell products, services and integrity. Customer first, quality first, quick response, continuous improvement. Different models have different prices, and different process materials have different prices.
| Model No. | VTS-1 | Material Rubber | Plastic |
| Certificate | CE | Reactant State | Liquid |
| Trademark | JSHXRT | Specification | 1test/box |
| Origin | Taizhou | Volume | 2ml |

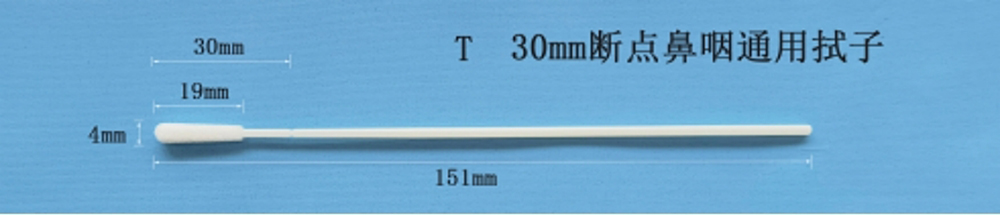

Model/A type: 1/pack, 100/pack, 200/pack, 500/pack.
Type B: 1/bag, 100/bag, 200/bag, 500/bag.
Intended use Disposable sampler for collecting, transporting and storing samples (virus, chlamydia, mycoplasma and ureaplasma).Indications: for the collection, storage and transportation of viruses, chlamydia, mycoplasma and ureaplasma.
Contraindications: This product is strictly prohibited for sampling bacterial samples, and the disinfectant itself contains antibiotics that inhibit bacteria.
Test population: suitable for all people who need to wipe
Target users: Qualified doctors, nurses collecting specimens in medical laboratories or hospitals
Composition: Usually consists of cotton swabs.
Scope of application: For sample collection, transportation and storage.
Precautions: This product is for single use only. The instructions for use must be strictly followed. It should be destroyed immediately after use. Reuse is prohibited. The disposal of its waste shall be carried out in accordance with the provisions of national environmental protection laws and regulations.
Storage requirements: The product should be stored in a dry, ventilated, radiant, clean room. The batch number of the product is on the package seal and is valid for 2 years. Please read the instruction manual carefully before use, and pay attention to the production date and batch number.
Instructions for use:
1. Sampling tubes and swabs can be sterilized before use by medical institutions or users.
2. Mark the relevant sample information on the sampling tube and inject an appropriate amount of sample solution.
3. According to different sampling requirements, collect the sample swab at the corresponding position, hold the handle and gently insert the swab into the sampling area, gently rotate the swab 3-5 times, and then take it out slowly.
4. Put the sample into the sampling tube, remove the sample swab from the sampling tube, tighten the cap, seal the sample, and complete the sampling.
5. Newly collected clinical specimens should be sent to the laboratory within 48 hours at 4°C, and shall not be sent to the laboratory within 48 hours. They should be kept at or below -70ºC. Specimens sent to the laboratory should be inoculated and separated as soon as possible, and stored at 4°C for 48 hours. If the inoculum is not inoculated, it should be kept at -70 ºC or below.
6. The conventional sampling method is as follows:
A) Nasal Swab: Gently wipe the tip of the swab into the nasal passages of the nasal passages, stay for a while, and then slowly exit. Wipe the other nostrils with another cotton swab, dip the tip of the swab into the sample, and discard the tail.
B) Pharyngeal swab: wipe the bilateral pharyngeal tonsils and posterior pharyngeal wall with a swab, immerse the swab head in the sampling solution, and discard the tail.
C) Gargle: gargle with 10mL normal saline, rinse the head and back, make an "oh" sound, let the saline rotate in the pharynx, and collect the wash with a 50mL empty sampling tube.
D) Nasal washing: The patient takes the body position, tilts the head slightly back, and injects 50 mL of normal saline into the nostrils with a pipette. He asked the patient to make a K sound to close the pharynx at the same time, and then asked the patient to lower his head to release the normal saline, collect the lotion with a 50mL empty sampling tube, and repeat the above process to clean both nostrils.
E) Nasopharyngeal aspirate: Tracheal and bronchial secretions are typically collected with this method. Mucus is extracted from the nasopharynx using a collector connected to a negative pressure pump. First insert the collection head into the nasal cavity and turn on the negative pressure. The head of the collector is collected and pushed out slowly. To collect mucus, wash the collector 3 times with 5 mL of liquid.
F) Autopsy specimens: collect autopsy tissue specimens, and perform specimen separation if necessary. Specimens are autopsy tissue.
G) Mycoplasma, Chlamydia and Ureaplasma urealyticum Ureaplasma urealyticum samples: Male: Rotate sterile cotton around the urethra to deflate for 1 minute.
2 cm for a few seconds. Women: To remove mucus from cervical mucus, insert 1-2 cm into the cervical canal with a sterile cotton swab.
 JIANGSU HXRT MD CO., LTD. is located in 1-A2 Yangfan Pioneer Park, the Modern Science and Technology Industrial Park, Jiangyan District, Taizhou City, Jiangsu Province, China. It is an enterprise specialized in manufacturing disposable medical plastic ware. With unremitting effort, the Company has come a long way and accumulated rich practical experience.
JIANGSU HXRT MD CO., LTD. is located in 1-A2 Yangfan Pioneer Park, the Modern Science and Technology Industrial Park, Jiangyan District, Taizhou City, Jiangsu Province, China. It is an enterprise specialized in manufacturing disposable medical plastic ware. With unremitting effort, the Company has come a long way and accumulated rich practical experience.HUAXIARUITAI always treats the product quality as vitality. Following the International standards, we has established a whole set of strict inner quality control system and passed ISO9001,ISO13485, and CE,FDA approval. Good quality and stability guarantees the testing data accurate and effective. Meanwhile, competitive price has made HUAXIARUITAI's products preferred in the medical market.
Under the marketing concept of humanistic service, HUAXIARUITAI keeps on doing the primary technology R&D, with a view to the filed of life sciences, gene organization and cell engineering, etc. In the meantime of increasing technology content, HUAXIARUITAI also applies for many invention and new-style utility patents, which is enhancing his core competence step by step.
Advanced technical equipment and systematic training for the staff continuously improve HUAXIARUITAI's manufacturing technique and service quality for clients. We will strive to sincerely cooperate and make progress together with all the clients just as in the past, to make due contributions to human medical and health services.



Video
Send your inquiry to this supplier



















