1 / 1
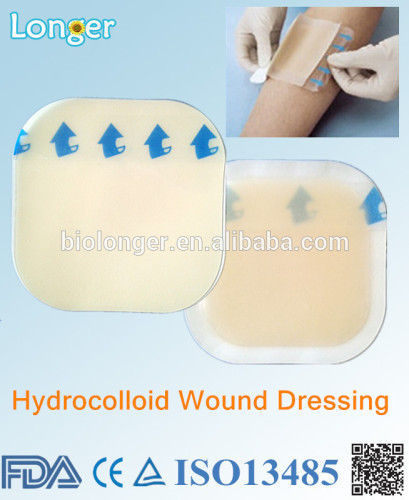
Hydrocolloid wound dressing with CE FDA in Hangzhou.
Get Latest Price
Send Inquiry
| Model No. : | M |
|---|---|
| Brand Name : | Longsun |
Hangzhou Qiandao Lake Longer Biotechnology Co., Ltd.
You might also like
Product description
Product Description Hydrocolloid wound dressing with CE FDA in Hangzhou Hydrocolloid dressing action principle and advantage: 1 It is conducive to the activity of collagenase, accelerate necrotic tissue dissolution, realize the debridement. 2 moisture and water absorstion co-exist, hydrocolloid belongs to semi permeable membrane, maintain high humidity with wound surface , can be formed with epithelial tissue, promote wound healing; also can absorb exudate through fluid much, is not conducive to bacterial growth. 3 insulation resistance, so that the wound is kept at a constant temperature, contribute to the formation of granulation tissue and strengthening Phagocytes; 4 Bacteria can not go through the water film, prevent bacteria and other objects get into, to reduce infection; occlusive dressings, make odor will not stick to the wound when removed, not easy to damage in neonatal capillary and granulation tissue, has certain elasticity, good compliance 5 Moisturizing dressing make patient comfortable, also has good elasticity, can be used for a long time, reduce the replacement times, reduce the pain and anxiety of patients when change dressing 
 [Sterilization method] the product sterilization by gamma ray sterilization [Applicatiopn Department] for operation room [Scope of application] applicable to all types of low to moderate exudative wounds. 1, Used after operation 2, All kinds of superficial trauma and cosmetic beauty; 3, the small area burn; 4, Chronic wound granulation and epithelial formation stage; 5, Donor site wound; 6, one and two period bedsore; 7, Venous leg ulceration [Useage Methods] 1, The operation wound were sterilized before use 2, Select the appropriate specifications; 3, open the package and take off the release paper paste and then dress to affected area. [note] 1, the product is aseptic packaging, damaged package shall be disabled 2, the product is disposable, only used within validity period, timely destructed after use. 3, production batch number and validity time could be find on the package; 4, Keep storage cool, ventilation, drying, guard against damp. [Contraindication] general contraindications this product without side effects, patients who have the special history of allergy of skin of patients shall be with caution [Specisification]
[Sterilization method] the product sterilization by gamma ray sterilization [Applicatiopn Department] for operation room [Scope of application] applicable to all types of low to moderate exudative wounds. 1, Used after operation 2, All kinds of superficial trauma and cosmetic beauty; 3, the small area burn; 4, Chronic wound granulation and epithelial formation stage; 5, Donor site wound; 6, one and two period bedsore; 7, Venous leg ulceration [Useage Methods] 1, The operation wound were sterilized before use 2, Select the appropriate specifications; 3, open the package and take off the release paper paste and then dress to affected area. [note] 1, the product is aseptic packaging, damaged package shall be disabled 2, the product is disposable, only used within validity period, timely destructed after use. 3, production batch number and validity time could be find on the package; 4, Keep storage cool, ventilation, drying, guard against damp. [Contraindication] general contraindications this product without side effects, patients who have the special history of allergy of skin of patients shall be with caution [Specisification]
Notes: We could change size according to your requirement. Company Information Hangzhou Qaiandao Lake Longer Biolotechnology CO,.LTD lies in the international garden city-- Qiandao Lake Town. We concentrate on researching, developing and manufacturing bio-materials. The company is listed as the key supported enterprise by Hangzhou Science Developing Department. The company maintain "Elaborated manufacture and sincere services" business purposes, insisting on high starting point, high standard and high qualification. We are developing with innovation,qualifying ourselves with strictness, winning our customers with sincere service and we will continue developing and bringing new products and projects, providing clinics world class service. Meanwhile, we values the most the brand building and we have forged the customers-believing brand of LongSun a steady platform of bioengineering, with superior products, reliable quality, qualified after-sale services and total customer satisfaction. 

 [Sterilization method] the product sterilization by gamma ray sterilization [Applicatiopn Department] for operation room [Scope of application] applicable to all types of low to moderate exudative wounds. 1, Used after operation 2, All kinds of superficial trauma and cosmetic beauty; 3, the small area burn; 4, Chronic wound granulation and epithelial formation stage; 5, Donor site wound; 6, one and two period bedsore; 7, Venous leg ulceration [Useage Methods] 1, The operation wound were sterilized before use 2, Select the appropriate specifications; 3, open the package and take off the release paper paste and then dress to affected area. [note] 1, the product is aseptic packaging, damaged package shall be disabled 2, the product is disposable, only used within validity period, timely destructed after use. 3, production batch number and validity time could be find on the package; 4, Keep storage cool, ventilation, drying, guard against damp. [Contraindication] general contraindications this product without side effects, patients who have the special history of allergy of skin of patients shall be with caution [Specisification]
[Sterilization method] the product sterilization by gamma ray sterilization [Applicatiopn Department] for operation room [Scope of application] applicable to all types of low to moderate exudative wounds. 1, Used after operation 2, All kinds of superficial trauma and cosmetic beauty; 3, the small area burn; 4, Chronic wound granulation and epithelial formation stage; 5, Donor site wound; 6, one and two period bedsore; 7, Venous leg ulceration [Useage Methods] 1, The operation wound were sterilized before use 2, Select the appropriate specifications; 3, open the package and take off the release paper paste and then dress to affected area. [note] 1, the product is aseptic packaging, damaged package shall be disabled 2, the product is disposable, only used within validity period, timely destructed after use. 3, production batch number and validity time could be find on the package; 4, Keep storage cool, ventilation, drying, guard against damp. [Contraindication] general contraindications this product without side effects, patients who have the special history of allergy of skin of patients shall be with caution [Specisification] | No. | Size | PKG |
| C21-1515 | 15*15cm | 400PCS/BAG |
| C21-1010 | 10*10cm | 400PCS/BAG |
| C21-0505 | 5*5cm | 600PCS/BAG |

Send your inquiry to this supplier
Send Inquiry